What is it?
Acid rain is like normal participation that can occur in many forms such as snow, fog, hail, rain, etc. , but it contains high levels of nitric and sulfuric acids. It can also come in two main forms; dry deposition and wet deposition. Wet deposition is when acidic rain forms in wet structures such as rain, snow, etc. which is most commonly seen. When it comes in dry position, the acidic particles in the air mix with other gases making a moisture. Soon the moisture attaches to some type of surface (such as a person, food, pet, building, soccer ball,etc.), as for a human or pet this can lead to some health problems. As soon as the acidic rain washes off the object and onto the ground it can harm the surface of the ground containing plants and other animals.https://www.epa.gov/acidrain/what-acid-rain
How is it made?
Acid rain occurs when sulfur dioxide (SO2) and nitrogen oxides (NOX) make chemical reactions in the atmosphere and then carried around by air currents causing it to become dry/wet deposition acidic rain. The sulfuric and nitric acids in the air are created when SO2 and NOX mix with water, air, dirt, and other chemicals.
https://www3.epa.gov/acidrain/education/site_students/whatcauses.html
What causes it to happen?
Most acidic rain comes from pollution made by human activity like power plants and factories. When humans burn fossil fuels such as coal, gas, and oil it releases the sulfuric and nitric oxides into the atmosphere. This isn't the only way, also running cars and buses release these oxides into the air, and if there is a lot of it, there is most likely a forecast of acid rain soon.
this photo describes the process acid rain goes through in order for it to be created https://www.epa.gov/acidrain/what-acid-rain
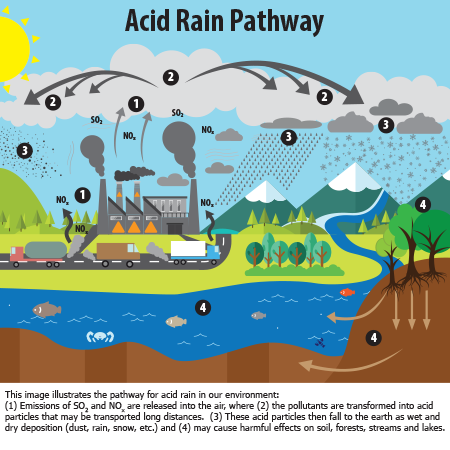
What effects does it leave on us and the environment?
If a human inhales acid rain or even swims in a lake with acidic rain or runs in acid rain (without knowing) it can cause health problems such as lung and heart disorders, bronchitis, and asthma. https://www.epa.gov/acidrain/effects-acid-rain Acid rain is also very harmful to many types of ecosystems and the wildlife and plants living within them. For example when acid rain rains in a forest, the acids fall into the ground and mix in the soil. The sulfuric and nitric oxides harm the growing of trees,bushes,etc. because the oxides dissolve the nutrients and calcium in the ground that is used to help trees grow and give life to the forest (and other plants and animals). Or if the acid rain falls into the lake it can harm humans who decide to go swim in it because they inhale the air around the lake, but also if they might accidentally swallow the water. It can also hurt the animals and plants in or around the lake, although it was discovered that some animals are tolerable around acidic rain, but many aren't. When acid rain appears frequently in the environment it can cause a lot of problems. The grass and bushes won't grow too much and won't have enough nutrients, and when animals want to feed on food they find not find any because there isn't so much food. In a lake or river, some fish can survive acidic rain, but most adult fish die and with acidic rain in the water most fish eggs can't even hatch because of the high levels of acids. Lack of nutrients and food for species of animals, or a decreasing population seen in the species can effect the food chain. https://www.epa.gov/acidrain/effects-acid-rain
this photo gives examples of animals that can tolerate acidic rain only to specific pH levels https://www.epa.gov/acidrain/effects-acid-rain

How is acidic rain measured?
When people measure the acidity of the acid rain they use the pH meter, which determines how acidic or how basic (alkaline) an acid or base is. The scale goes from 0-14, any numbers from 0-6/7 means that it is an acid (0 being the strongest), 7 is neutral meaning it's neither very acidic or very basic, numbers from 8-14 means that it is a base (14 being the strongest). Unpolluted rain will have a pH between 5 and 6, so it cannot really harm you, and acid rain has a pH of about 4.
http://webprojects.oit.ncsu.edu/project/bio183de/Black/chemreview/chemreview_reading/acid_rain.html
here is a pH scale determining strong alkalines and acids and the neutral types and a few examples of some below. https://www.epa.gov/acidrain/what-acid-rain

What are people doing to help this problem?
Scientists are finding ways of reducing the amount of sulfur and nitric oxides being released while burning fossil fuels. A suggestion that was made was burning coal that contains less sulfur, also many power plants now have special machines called scrubbers that eliminates the sulfur oxide in the leftover gases in the smokestack. Also some power plants are changing the way they burn coal, because the way nitrogen oxides are created is during the process of burning coal. Many governments are also trying to spend more of their money on making solutions or remedies to pollution problems like this one. Fossil fuels make energy, as well as acid rain, so people nowadays try to use wind and solar power instead of burning fossil fuels. Car manufacturers also try to put catalytic converters in their cars because it reduces the amount of sulfuric and nitric oxides and other pollutants coming out of the car. https://www3.epa.gov/acidrain/education/site_students/beingdone.html



